Siffofi
Ana auna na'urar lantarki ta ion ta yanar gizo a cikin ruwan da aka yi amfani da shi wajen tattara sinadarin chlorine ko kuma tantance iyaka da kuma nuna sinadarin fluorine/chlorine a cikin electrode domin samar da hadaddun sinadaran da ke cikin sinadarin ion.
| Ka'idar aunawa | Potentiometry na zaɓin ion |
| Kewayon aunawa | 0.0~2300mg/L |
| Zafin jiki ta atomatikkewayon diyya | 0~99.9℃,da 25℃ kamar yaddayanayin zafi na tunani |
| Matsakaicin zafin jiki | 0~99.9℃ |
| Zafin jiki ta atomatikdiyya | 2.252K,10K,PT100,PT1000 da sauransu |
| An gwada samfurin ruwa | 0~99.9℃,0.6MPa |
| Ion ɗin tsangwama | AL3+,Fe3+,OH-da sauransu |
| kewayon ƙimar pH | 5.00~10.00PH |
| Babu komai a ciki | > 200mV (ruwan da aka cire ion) |
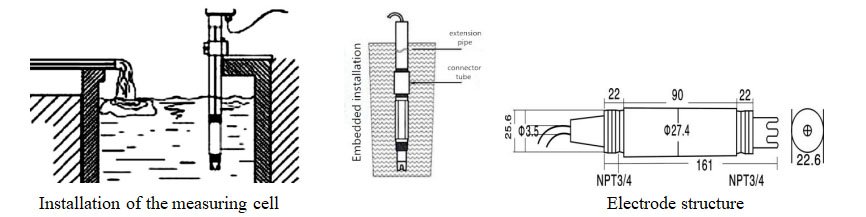
| Tsawon lantarki | 195mm |
| Kayan aiki na asali | PPS |
| Zaren lantarki | Zaren bututu 3/4(NPT) |
| Tsawon kebul | Mita 5 |
Ion wani atom ne mai caji ko molecule. Ana cajinsa ne saboda adadin electrons bai kai adadin protons a cikin atom ko molecule ba. Atom na iya samun caji mai kyau ko kuma caji mara kyau dangane da ko adadin electrons a cikin atom ya fi ko ƙasa da adadin protons a cikin atom.
Idan kwayar zarra ta jawo hankalin wani kwayar zarra saboda ba ta da adadin electrons da protons da suka yi daidai, ana kiran kwayar zarra da ION. Idan kwayar zarra ta ƙunshi electrons da yawa fiye da protons, to kwayar zarra ce ko kuma ANION. Idan kwayar zarra ta ƙunshi protons da yawa fiye da electrons, to kwayar zarra ce mai kyau.















